Why Are Toxic Phthalates Still Used in Medical Supplies for Premature Babies?
 By
By
Since the late 1960s research has shown that a plastic additive in polyvinyl chloride (PVC), leaches from medical devices and is toxic to multiple organs, especially for premature infants.
Despite more than two decades of evidence, advocacy and education around the issue, PVC products containing this harmful phthalate chemical still dominate the neonatal intensive care unit (NICU) environment.
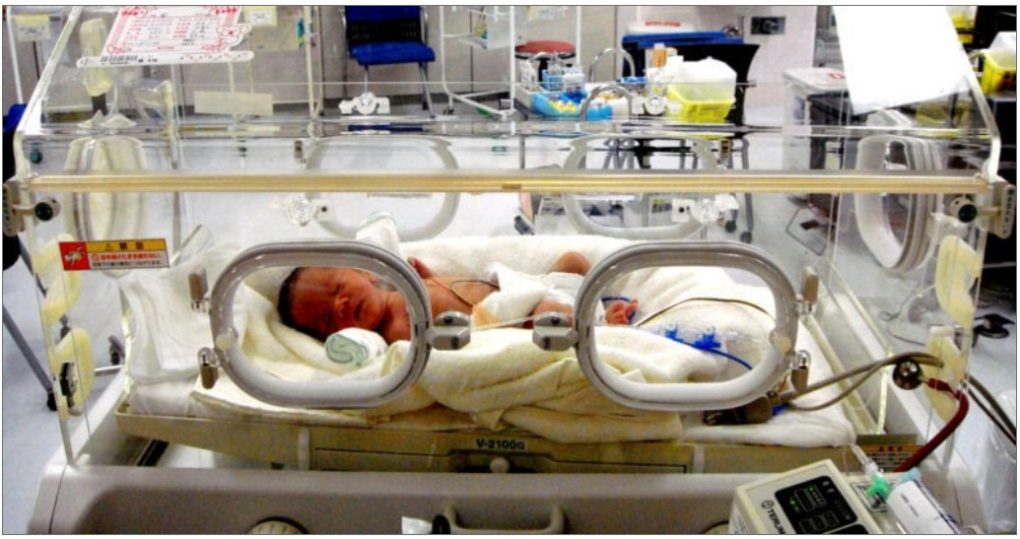
Feeding tubes, fluid bags, syringes, respiratory support tubes, intravenous lines, nasal cannulas, catheters, incubators — this is only a short list of the PVC medical supplies that assist in everything from eating, to breathing, to sleeping for premature infants in NICU.
The majority of these devices contain DEHP, or Di-2-ethylhexyl phthalate, a class of chemicals called phthalates, which are used to make plastic softer and more flexible.
Phthalates mimic the body’s hormones and can disrupt important processes during an infant’s rapid development.
Scientists have linked phthalate exposure for newborn infants, also known as neonates, with several toxic endpoints including damage to the developing brain, liver, heart, lungs, male reproductive tract and more.
While training as a clinical neonatology fellow and pursuing a masters of public health in the early 2000s, Dr. Annemarie Stroustrup Smith, the vice president and director of neonatal services at Northwell Health in New York, started to draw connections between the emerging research on prenatal phthalate exposure and the health outcomes observed among premature infants.
“We tend to chalk up health challenges that children born preterm have as due to prematurity, but that’s not really a mechanism,” Stroustrup Smith told Environmental Health News, “So my question was, are some of those [health challenges] due to phthalate exposure? And if it is, that’s something we can fix because we totally control the NICU environment.”
Stroustrup Smith’s research adds to a growing body of studies seeking to understand levels of neonatal exposure to DEHP, health effects and the benefits and drawbacks of alternatives.
And the science is making a difference — there is positive movement in the marketplace with phthalate-free devices becoming increasingly available.
However, cost remains an issue and the contaminated medical devices continue to fall through the regulatory cracks.
Lack of medical device regulation
Some DEHP-free medical supplies, such as feeding tubes, are readily available on the market. However, it is impossible to have a completely phthalate-free NICU in the U.S. due to unavailability and the high cost of alternative options.
While the U.S. Food and Drug Administration (FDA) released a guidance document for the pharmaceutical industry on avoiding DEHP in 2012, they have yet to ban or restrict its use in medical supplies, like the European Union has done.
This is despite ongoing research, advocacy and a direct ask from members of Congress who wrote a letter to the FDA last year.
“Patients should not be exposed to phthalates and [endocrine-disrupting chemicals] when they seek medical treatment,” the representatives wrote in a letter to acting FDA chief Dr. Janet Woodcock.
Natural Blaze is Google Free!
Support Independent Media for as Little as $5 Per Month
According to Joel Tickner, a chemical policy expert and professor at the University of Massachusetts Lowell, two big reasons industry hasn’t switched from DEHP are cost and resistance to change, but regulation would solve that. “It’s policy,” he told Environmental Health News, “if the FDA put their foot down and said, ‘we need to move in the next five years,’ that would change things very quickly.”
The FDA says that DEHP is on their radar and they issued a discussion paper last year for the public and stakeholders to comment.
However, the paper does not specifically mention DEHP. In addition, the FDA only approves devices in their final form.
“The FDA does not clear or approve individual materials that are used in the fabrication of medical devices, but does take the chosen components and materials into consideration,” FDA media representative Audra Harrison wrote to Environmental Health News.
Market-based medical device solutions
The international organization Healthcare Without Harm started working with researchers in the late 1990s to raise awareness about phthalate exposure in the NICU.
Today, their sub-organization Practice Greenhealth focuses on leveraging the purchasing power of more than 1,500 healthcare organizations in their network and helping health systems make informed purchases.
“At the end of the day, I think the most expedient and long-lasting impact is a market-based solution,” John Ulman, director of safer chemicals and procurement at Healthcare Without Harm, told Environmental Health News.
For example, In 2012, Kaiser Permenante, one of the largest U.S. healthcare companies, switched to non-DEHP and non-PVC IV bags.
According to Seema Wadhwa, the executive director for environmental stewardship, Kaiser made this switch in six months, including product performance testing, and saved $5 million in annual costs. In 2021, the medical supplier B. Braun launched CARESAFE, the first PVC-and-DEHP-free IV sets on the U.S. market.
The three-year process from development to launch was rigorous and resource-intensive, requiring creative engineering, process validation, testing and FDA clearance.
“Four decades ago we recognized environmental and safety risks from DEHP and PVC,” Scott Moyer, the associate director of research and development at B. Braun, told Environmental Health News. “The goal is from the bag to the patient making sure that pathway is free from harmful chemicals overall.”
In order to bolster the market around safer NICU medical devices, Stroustrup Smith said researchers need more data to prove to clinicians that switching materials will improve infant health outcomes, which takes time.
“If you look at making changes in medical care, typically from the first point an intervention is shown to be effective, it often takes a decade before you actually get that change,” she said, “and that’s when it’s a slam dunk, totally obvious visible change…this is not that straightforward.”
Key considerations for swapping phthalate-contaminated medical devices
All DEHP substitutions are not created equal. “We have to be wary of regrettable substitutions,” said Ulman when describing the dangers of replacement chemicals that are not well studied and could have similar effects.
For example, some alternative plasticizers, such as DINCH, have thorough toxicological data, but others have little to none.
Some experts argue that the material itself, PVC, is problematic and that instead of swapping DEHP for another plasticizer, manufacturers should switch to materials that don’t require plasticizers.
The entire life cycle of PVC is harmful — production requires a lot of energy and releases toxic chemicals such as mercury and asbestos into water and air.
For disposal, PVC is the least recyclable plastic and is often incinerated by healthcare facilities, creating highly toxic and persistent pollutants called dioxins and furans.
For this reason, PCV-free materials, such as the thermoplastic polyurethane in B. Braun’s CARESAFE line, are the preferred substitutes.
Toward phthalate-free
Outside of medical supplies, phthalates are found in a wide range of products including building materials, cosmetics, furniture, food packaging and more.
Thus, there are several opportunities across a person’s lifespan to come into contact with phthalates, starting from the womb.
A 2022 study linked prenatal phthalate exposure to an increased risk of preterm birth — meaning there is a chance infants born preterm due to phthalate exposure are then exposed to even more phthalates in the NICU.
Since scientists first raised concern about DEHP, progress towards reducing exposure to children and infants in the U.S. has inched along.
In 2008, Congress banned DEHP and two other phthalates in toys and in 2017 the U.S. The Consumer Product Safety Commission banned five additional phthalates in toys.
Prominent health organizations, such as the American Public Health Association and the American Academy of Pediatrics have published policy statements on the issue.
NICU’s across the country have committed to buying DEHP-free products whenever possible.
Individuals can also play a role.
Healthcare professionals can advocate for DEHP-free products with healthcare administration, researchers can continue to study the impact of DEHP exposure and the benefit of replacements, and patients can ask their doctors about exposure to phthalates during care.
Change takes time, but some argue that we shouldn’t wait to act on protecting the most vulnerable patients. “The science was there 20 years ago,” Tickner said, “Why is it taking so long to act on this?”
Originally published by Environmental Health News.
Ashley James is a reporting intern for Environmental Health News and an ORISE Fellow for the U.S. EPA, Office of Children’s Health Protection.
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the views of Children’s Health Defense.
Sourced from Children’s Health Defense
Photo credit: Tamaki Sono/flickr